H20 Is An Example Of
If you want a detailed guide on the covalent bonding of water (H2O), you will honey this amazing guide I am about to share with you.
Simply first of all, permit's offset with a simple introduction.
In Chemical science, a covalent bail is formed due to the sharing of electrons between atoms. Only, why does this "sharing of electrons" take place?
Let me explain. But earlier that, allow'southward talk near covalent bonding in detail.
What is Covalent Bonding?
In this type of bonding, the "participating" atoms share electrons with each other to become stable.
Here is what I hateful.
In an outermost shell, an cantlet should have two or eight electrons to accomplish the atomic configuration of a noble gas.
Noble gases are unreactive (as they have fully-filled outer shells). So, whenever an atom becomes stable, it is said to take achieved the electronic configuration of a noble gas.
Pretty simple, isn't it?
Important Note: At this stage, you might be wondering, why not atoms commutation electrons like ionic bonding? Why does this commutation of electrons take place?
The answer is unproblematic, ionisation energy (energy essential to take away an electron).
Elements that have high ionisation free energy cannot transfer electrons. Plus, the elements having a low electron affinity cannot take upwardly electrons.
Every bit a result, the atoms of such elements share electrons with each other (to accomplish stability).
The 2 ways in which covalent bonding tin be achieved:
- The sharing of electrons between atoms of different elements (for example Water, CH4 and NH3 etc).
- The sharing of electrons betwixt atoms of the same elements (for example N2, H2 and O2 etc).
If I talk about water (H2O), we will be talking about the first method.
Covalent Bonding of Water (H2o):
Here's how it takes identify.
If I talk almost hydrogen ( H1 ), it has one electron in its outermost shell. To become stable, the hydrogen cantlet needs one electron to accomplish a duplet electronic configuration (the duplet rule).
The Hydrogen cantlet follows the duplet rule that says: An cantlet is stable if it has two electrons in its outer shell.
Moving on, the Oxygen atom ( O8) has six electrons in its outer trounce. Therefore, it needs ii more electrons to get stable (achieve octet electronic configuration).
The Oxygen cantlet follows the octet rule in which the atom completes viii electrons in its valence (outermost) shell.
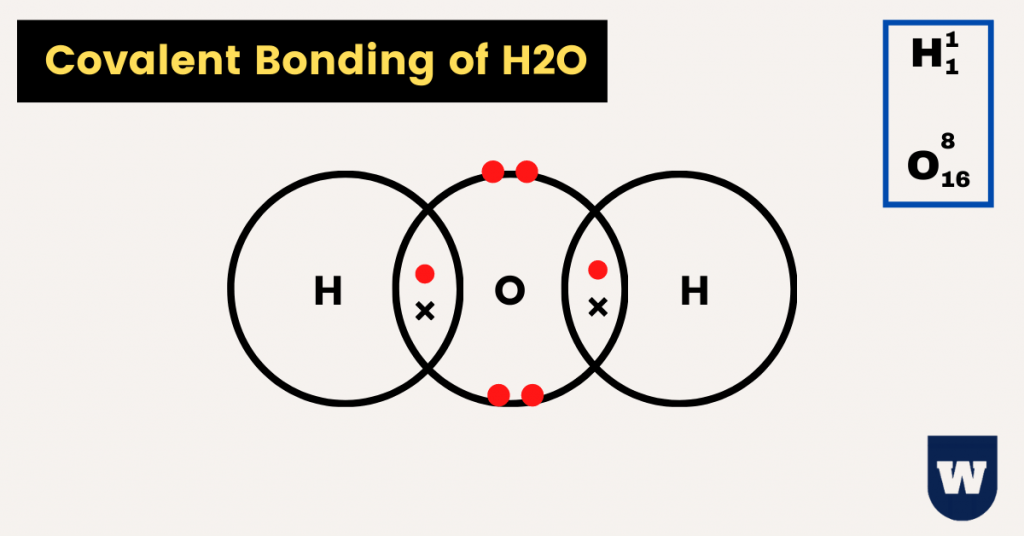
Equally you can see in the paradigm in a higher place, two hydrogen atoms and one oxygen atom share electrons with each other to become stable.
Water is a polar covalent molecule. This is because of the unequal sharing of electrons (due to the deviation in electronegativity) between oxygen and hydrogen.
In short, hither is the summary.
- An oxygen atom has six electrons and needs 2 more electrons (co-ordinate to the octet rule).
- A hydrogen cantlet has 1 electron and needs one more electron (co-ordinate to the duplet rule).
- Every bit a result, the oxygen cantlet shares electrons with 2 hydrogen atoms. This results in the germination of a water molecule (H2O).
To further sympathize this topic, let'southward have a look at another example to understand this concept.
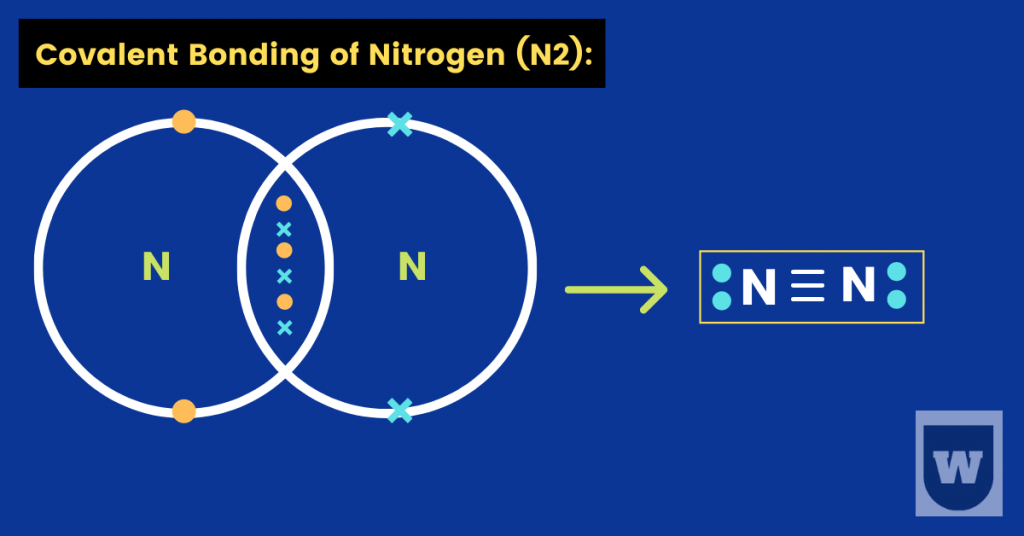
Example: Covalent Bonding of Nitrogen:
This case volition assistance you empathize this topic in a better mode.
If I talk about the Nitrogen cantlet (Northward7 ), it has seven electrons in full (five in its valence shell). This means that a nitrogen atom needs three more electrons to become stable.
As a result, 2 Nitrogen atoms share electrons with each other.
In elementary words, a nitrogen atom can fill its octet by sharing three electrons with another nitrogen atom. This results in the formation of three covalent bonds, a then-called triple bail.
Pretty simple, isn't it?
Further reading:
Ionic Bonding of sodium chloride (NaCl) | All-time Guide
Electrolysis Made Unproblematic | Notes
Exothermic and Endothermic reactions | Complete Guide
With this, it is time to move on and talk about the properties of covalent compounds.
Properties of covalent compounds:
Here are the iv properties that you should know:
- Covalent bonding involves neutral molecules (having no electric charge).
This is the reason that forces of allure between these molecules are weaker as compared to ionic compounds. Therefore, these compounds are normally volatile gases or liquids.
- Covalent compounds (generally) have depression melting and humid indicate.
Let's recall from the showtime property.
Equally I mentioned higher up, these compounds are made upwardly of neutral molecules. Plus, the forces of allure between these molecules are weak.
So, a low amount of heat is required to break these bonds. This explains why they have a low melting and boiling point.
Note: Since the intermolecular forces are weak, covalent compounds are as well soft and brittle (considering they break or distort with force).
Now, let me tell y'all about the 3rd holding.
- Covalent compounds practise not bear electricity.
I have a question for you. What is the basic requirement to conduct electricity?
Ions or electrons.
And if you recall, covalent compounds involve neutral molecules. Since there are no free moving ions or electrons, these compounds do not acquit electricity.
In other words, these compounds do not take charged particles to ship electrons.
Before talking about the next property, let me tell you about polar and non-polar covalent bonds.
In Nonpolar covalent compounds, the electrons are shared as between the two atoms. For example: In the covalent bonding of Hydrogen (H2), both hydrogen atoms share one electron with each other.
And, Nonpolar compounds are stronger.
On the other hand, polar compounds are different.
A polar compound is one in which the sharing of elections (or the allure of electrons) is unequal.
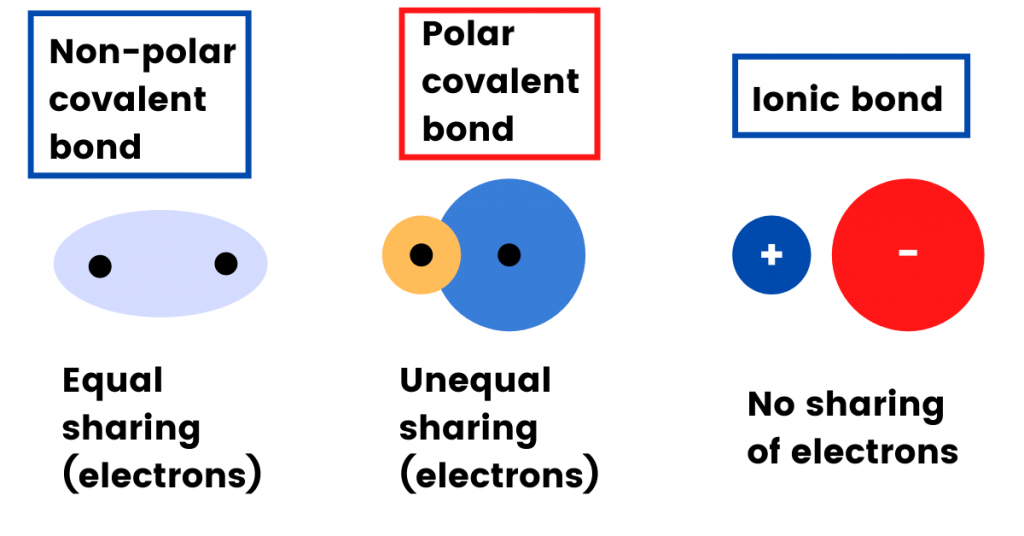
An case is the hydrogen chloride molecule (HCl). The bonding between hydrogen and chlorine leans more towards chlorine atoms.
This is because chlorine (Cl) is more electronegative in nature as compared to hydrogen.
- Covalent compounds are soluble in organic solvents but insoluble in water.
Hither'south why.
Remember that most of the organic solvents are not-polar in nature. And, covalent compounds are as well (mostly) non-polar.
Since "like dissolves like", these compounds are soluble in organic solvents.
And, almost of the covalent compounds are insoluble in water.
Since h2o is a polar solvent, it does not dissolve Nonpolar compounds. Plus, when covalent compounds are dissolved in h2o, they dissociate into molecules (only not into ions).
This takes us straight to our adjacent topic, types of covalent bonds.
Types of Covalent Bonds:
The covalent bonds, based on the number of shared pairs of electrons, can exist classified every bit:
- Single covalent bond
- Double covalent bond
- Triple covalent bail
Allow's take a look at each of them in detail.
Single bonds:
If only one pair of electron is shared between the 2 "participating" atoms, then we say that a unmarried bond is formed.
In other words, but two electrons are shared in this type of bail.
Note: This is the virtually stable type of bond but has less density (and is also weaker) than double and triple bonds.
For case, hydrogen chloride (HCl).
A hydrogen cantlet has 1 valence electron while a chlorine atom has seven valence electrons. As a result, a single bail is formed between the hydrogen and chlorine atom past sharing one electron.
Double bond:
When ii pairs of electrons are shared, we say that a double bail is formed. This type of bail is represented past two dashes (=).
As I mentioned above, this bond is much stronger but less stable than a single bond.
For example, carbon dioxide (CO2). The structural formula is: O=C=O
The carbon atom has four valence electrons while the oxygen atom has six valence electrons. So, each oxygen atom shares 2 electrons with two electrons on the key carbon atom.
And then this forms a double bond.
Triple bail:
If three pairs of electrons are shared, nosotros say that a triple bond is formed. This type of bond is represented by three dashes (≡).
You lot should know that these are the least stable types of covalent bonds.
An case is the Nitrogen molecule (N2).
A nitrogen cantlet has five valence electrons and it needs three more than electrons to get stable. And so in the formation of a nitrogen molecule, each nitrogen cantlet shares three electrons.
Therefore, a triple bond is formed.
Covalent Bonding vs Ionic Bonding:
When studying this topic, it is important to talk about this question as well.
Well, what is the difference betwixt an ionic and covalent bond?
A covalent bail is formed between non-metals where sharing of electrons takes identify. Simply in ionic bonding, cation (positive ion) and anion (negative ion) are involved where transfer of elections takes place.
Here is some detail for yous.
An ionic bond is a type of chemical bail in which the atoms have different electronegativity values from each other. For instance, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table table salt).
However, in a covalent bond, the atoms are bound to share electrons. For case, if we talk about water (H2o), information technology is a polar covalent bail. This is considering of the unequal sharing of electrons.
Here is a simple table for you to empathise this topic in a amend way:
| Ionic Bonds | Covalent Bonds | |
| Description | Transfer of electrons | Sharing of electrons |
| Polarity | High | Depression |
| Melting & Boiling point | High | Low |
| State at room temperature | Solid | Liquid or Gas |
| Example | H2SO4, NaCl | Water, N2 |
FAQ's:
Here are some of the questions related to this topic.
Why is water covalently bonded?
Covalent bonding occurs between non-metals. Since both hydrogen and oxygen are non-metals, a covalent bail is formed between them.
This is suggested by the formula of water – H2o. This shows that two hydrogen atoms and one oxygen atom share electrons with each other to go stable.
How many covalent bonds are in H2O?
Two covalent bonds.
The concept is simple. The oxygen atom shares a pair of electrons with each hydrogen atom. Since at that place are 2 hydrogen atoms, 2 covalent bonds are formed that hold the water molecule together.
Wrapping up:
With this, our topic virtually the covalent bonding of water (Water) has come to an cease.
At present, it is your turn.
Which function of the topic do you observe interesting? Is information technology related to cartoon the structure of water (Water), or is information technology almost the deviation between ionic and covalent bonds?
Either way, do allow me know.
If you have any questions, you can drib them beneath. Thank you for staying with me till the end. Stay tuned for more.
H20 Is An Example Of,
Source: https://blogswithwg.com/covalent-bonding-of-water/
Posted by: sheppardanstor47.blogspot.com


0 Response to "H20 Is An Example Of"
Post a Comment